What is corrosion?
Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide, hydroxide, sulfide, etc. It is the gradual destruction of materials (usually a metal) by chemical and/or electrochemical reaction with their environment.
Rusting, the formation of iron
oxides, is a well-known example of electrochemical corrosion. This type of
damage typically produces oxide(s) or salt(s) of the original metal and results
in a distinctive orange colouration. Corrosion can also occur in materials
other than metals, such as ceramics or polymers, although in this context, the term
"degradation" is more common.
Corrosion degrades the useful
properties of materials and structures including strength, appearance and permeability
to liquids and gases.
Why do Metals Corrode?
Metals corrode because we use them in environments where
they are chemically unstable. All metals exhibit a tendency to be oxidized,
some more easily than others. The driving force that causes metals to corrode
is a natural consequence of their temporary existence in metallic form. To
reach this metallic state from their occurrence in nature in the form of
various ores, it is necessary for them to absorb energy by smelting, refining
processes. These stored up energy is later returned by corrosion. Only the
precious metals (gold, silver, platinum, etc.) are found in nature in their
metallic state. All other metals are processed from minerals or ores into
metals which are inherently unstable in their environments and has the tendency
to return at its original state of ores.
Most of the cases corrosion occurs through oxidation and
reduction reactions.
Oxidation describes the loss of electrons by a molecule,
atom or ion
Reduction describes the gain of electrons by a molecule,
atom or ion
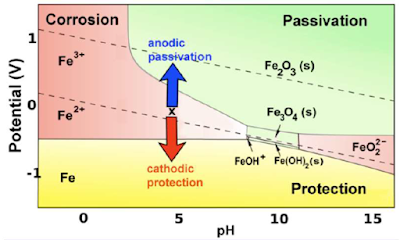
Pourbaix diagram for iron
A simplified Pourbaix diagram indicates
regions of immunity/protection, corrosion and passivity.
Corrosion of iron (and other
active metals such as Al) is indeed rapid in parts of the Pourbaix diagram
where the element is oxidized to a soluble, ionic product such as Fe3+(aq)
or Al3+(aq). However, solids such as Fe2O3,
and especially Al2O3, form a protective coating on the
metal that greatly impedes the corrosion reaction. This phenomenon is called passivation.
In the yellow part of the
diagram, an active metal such as iron can be protected by a second mechanism,
which is to bias it so that its potential is below the oxidation potential of
the metal.
This cathodic protection strategy
is most frequently carried out by connecting a more active metal such as Mg or
Zn to the iron or steel object (e.g., the hull of a ship, or an underground gas
pipeline) that is being protected.
The active metal (which must be
higher than Fe in the activity series) is also in contact with the solution and
slowly corrodes, so it must eventually be replaced.
In some cases, a battery or DC
power supply - the anode of which oxidizes water to oxygen in the solution - is
used instead to apply a negative bias.
Corrosive Environments:
- Air and humidity
- Fresh, distilled, salt and marine water
- Natural, urban, marine and industrial atmospheres
- Steam and gases, like chlorine
- Ammonia
- Hydrogen sulfide
- Sulfur dioxide and oxides of nitrogen
- Fuel Gases
- Acids
- Alkalies
- Soils
Corrosion may severely affect the following functions of metals, plant and
equipment:
(1) Impermeability: Environmental
constituents must not be allowed to enter pipes, process equipment, food
containers, tanks, etc. to minimize the possibility of corrosion.
(2) Mechanical strength: Corrosion
should not affect the capability to withstand specified loads, and its strength
should not be undermined by corrosion.
(3) Dimensional integrity:
Maintaining dimensions is critical to engineering designs and they should not
be affected by corrosion.
(4) Physical properties: For
efficient operation, the physical properties of plants, equipment and
materials, such as thermal conductivity and electrical properties, should not
be allowed to be adversely affected by corrosion.
(5) Contamination: Corrosion,
if allowed to build up, can contaminate processing equipment, food products,
drugs and pharmaceutical products and endanger health and environmental safety.
(6) Damage to equipment: Equipment
adjacent to one which has suffered corrosion failure, may be damaged.
Classification of Corrosion
Dry corrosion
• Corrosion
occurs in the absence of moisture.
• It involves
direct attack of dry chemicals/gases (Air and Oxygen) on the metal surface
through chemical reactions.
•This type
corrosion is not common and the process is slow.
• Corrosion
products are produced at the site of corrosion.
• The process of
corrosion is uniform.
Wet corrosion
• Corrosion
occurs in presence of conducting/aqueous media (strong or dilute, acidic or
alkaline) on metal through electrochemical reactions.
• It involves
formation of electrochemical cells.
• This type of
corrosion is quite common and it is a rapid process.
• Corrosion
occurs at anode but rust is deposited at cathode.
• It depends on the size of the anodic part of
metal.
Fig: Main forms of
corrosion attack regrouped by their ease of recognition
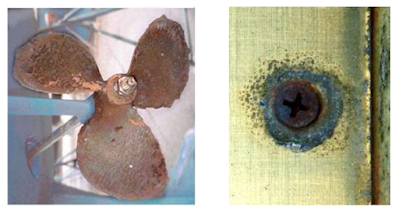
Uniform Corrosion
This is also called general
corrosion. The surface effect produced by most direct chemical attacks is a
uniform etching of the metal. Here, the corroded area is evenly distributed
across the material being attacked. Uniform corrosion can render large amount
of material useless quite rapidly because the attack occurs across the entirety
of the exposed surface.
As corrosion occurs uniformly
over the entire surface, it can be practically controlled by cathodic
protection, use of coatings or paints, or simply by specifying a corrosion
allowance.
In some cases uniform corrosion
adds color and appeal to a surface. Two classics in this respect are the
greenish patina created by naturally tarnishing copper and the rust hues
produced on weathering steels.
Besides, the breakdown of
protective coating systems on structures often leads to this form of corrosion.
Dulling of a bright or polished surface, etching by acid cleaners, or oxidation
(discoloration) of steel are examples of surface corrosion.
Cast irons and steels corrode
uniformly when exposed to open atmospheres, soils and natural waters, leading
to the rusty appearance.
The photos below are showing
uniform corrosion (rusting)
Galvanic Corrosion
Galvanic corrosion may
also be known as bimetallic corrosion or dissimilar metal corrosion.
It is an electrochemical action of two dissimilar metals in presence of an electrolyte and an electron conductive path. The driving force for corrosion is the potential difference between the different materials. In this corrosion process, one metal corrodes preferentially when it is acting as anode with respect to another which acts as cathode. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices.
Therefore, for
galvanic corrosion to occur, three conditions must all be present:
i.
Electrochemically dissimilar metals must be present.
ii. Metals must
be in electrical contact.
iii. Metals must be exposed to an electrolyte.
Main factors
influencing galvanic corrosion rates are:
i. Potential
difference between materials.
ii. Cathode
efficiency.
iii. Surface areas of
connected materials (area ratio).
iv. Electrical resistance
of the connection between the materials and of the electrolyte.
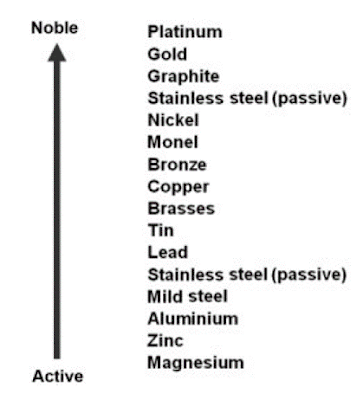
Basically, metals
and metal alloys possess different electrode potentials, a relative measure of
a metal's tendency to become active in a given electrolyte. The more active or
less noble a metal is, the more likely it will form an anode in an electrolytic
environment. While the more noble a metal is, the more likely it will form a
cathode when in the same environment.
The electrolyte
acts as a conduit for ion migration, moving metal ions from the anode to the
cathode. The galvanic series in seawater lists the common metals in order from
the most anodic to most cathodic (noble). The further apart the metals are in
this series, the greater the corrosion difference and speed between the two.
A tabulation of the relative electrochemical potential/
strength is called the galvanic series.
When galvanic cells are formed on different metals, the
galvanic corrosion occurs.
Why does corrosion cell form?
Metallurgical factors:
- Compositions.
- Microstructures.
- Inclusions.
- Precipitations.
- Heat treatment.
- Mechanical rolling and tempering.
- Welding.
- Work hardening.
- Fabrication, installation and external stress, strain factors.
Environmental factors:
- Concentration Cells.
- Environmental induced stress corrosion cracking (SCC), sulfide stress cracking (SSC), hydrogen-induced cracking (HIC), etc.
- Microbiologically Influenced Corrosion (MIC) - Microbial .
- Temperature induced corrosion.
- Mechanical environmental induced erosion, fretting, cavitation etc.
- Galvanic, CP and Impressed current anodic dissolution, stray current, cathodic embrittlement etc.
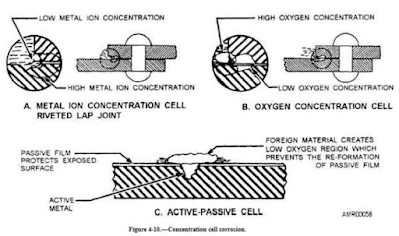
Concentration Cell Corrosion
This corrosion
occurs when two or more areas of a metal surface are in contact with different
concentrations of the same solution. It is the deterioration of parts of a
metal surface at different rates, due to the parts of the surface coming into contact
with different concentrations of the same electrolyte. The differing
concentrations result in some parts of the metal acquiring different electric
potentials. The extent of this corrosion reaction is proportionate to the
difference in concentrations at contact points. It also varies with the type of
electrolyte.
If an area of the
electrolyte close to the metal shows a lowered concentration of metal ions, the
region has to turn anodic in comparison to different portions of metal surface.
Thus, this part of the metal corrodes faster, so as to increase the local ion
concentration in electrolyte.
Concentration cell corrosion
is most prevalent in the presence of oxygen. When pure oxygen comes into
contact with a wet metal surface, corrosion action is enabled. However, the
corrosion is most severe in areas that have minimal oxygen contact. Parts of
metal that are covered by scale will corrode faster because the contact with
oxygen for these parts is restricted. Concentrated pitting can result due to
this cumulative reaction.
If a piece of
metal is immersed in an electrolyte and there is a difference in concentration
of one or more dissolved compounds or gases in the electrolyte, two areas of
metal in contact with solution differing in concentration will ordinarily
differ in solution potential, forming a concentration cell. Two electrically
connected pieces of a given metal could also form a concentration cell in the
same manner.
There are three general types of concentration cell corrosion:
1. Metal ion concentration
cells : In the presence of water, a high concentration of metal ions will
exist under faying surfaces and a low concentration of metal ions will exist
adjacent to the crevice created by the faying surfaces. The area of the metal
in contact with the low concentration of ions will be cathodic and will be
protected, and the area of metal in contact with the high ion concentration
will be anodic and corroded. Proper protective coating application with
inorganic zinc primers is also effective in reducing faying surface corrosion.
2. Oxygen concentration cells:
An oxygen cell can develop at any point where the oxygen in the air is not
allowed to diffuse uniformly into the solution, thereby creating a difference
in oxygen concentration between two points. Typical locations are under
metallic or nonmetallic deposits (dirt) on the metal surface and under faying
surfaces. Oxygen cells can also develop under gaskets, wood, rubber, plastic
tape, and other materials in contact with metal surface. Corrosion will occur
at the area of low-oxygen concentration (anode).
3. Active-passive cells:
Metals that depend on a tightly adhering passive film (usually an oxide) for
corrosion protection; e.g., austenitic corrosion-resistant steel, can be
corroded by active-passive cells. The corrosive action usually starts as an
oxygen concentration cell; e.g., salt deposits on the metal surface in the
presence of water containing oxygen can create the oxygen cell. If the passive
film is broken beneath the salt deposit, the active metal beneath the film will
be exposed to corrosive attack. An electrical potential will develop between
the large area of the cathode (passive film) and the small area of the anode
(active metal). Rapid pitting of the active metal will result.
Pitting Corrosion
It is a localized corrosion that occurs on a metal surface where there are intermetallics or microscopic defects related to very localised thinning or rupture of the natural oxide film. These sites are anodic with respect to their vicinity, and corrosion pits can develop due to electrochemical mechanism. The pits are also found underneath surface deposits caused by corrosion product accumulation. It occurs on mainly passivated metals and alloys in environments containing chloride, bromide, iodide or perchlorate ions when the electrode potential exceeds a critical value, the pitting potential. This form of corrosion is characterized by narrow pits with a radius of the same order of magnitude as, or less than, the depth. The pits may be of different shape, but a common feature is the sharp boundary. Pitting is one of the most destructive forms of corrosion as it causes equipment failures due to perforation / penetration. Moreover, pitting is dangerous since the material may be penetrated without a clear warning (because the pits often are narrow and covered) and the pit growth is difficult to predict. Moreover, pitting corrosion is difficult to measure because the number and size of pits (diameter and depth) vary from region to region and within each region. Short-term testing in the laboratory for determination of pit growth is also problematic because, under realistic conditions, it may take long time, e.g. many months, before the pits become visible. Another problem is that the critical size, i.e. the maximum pit depth, increases with increasing surface area.
There are two types of pits –
i) stable pits (those that
initiate immediately and then continue to grow in depth with time) and
ii) metastable pits (those that
may initiate late or that may eventually stop growing (‘die’) in depth).
Finding the deepest actual pit
requires a detailed inspection of the whole structure. As the area of the structure
inspected decreases, so does the probability of finding the deepest actual pit.
A number of statistical transformations are there to quantify the distributions
in pitting variables. Gumbel has developed the extreme value statistics (EVS)
for the characterization of pit depth distribution.
The EVS procedure is to measure
maximum pit depths on several replicate specimens that have been pitted, then
arrange the pit depth values in order of increasing rank. The Gumbel or extreme
value cumulative probability function F(x), is shown in Eq. 1, where λ and α
are the location and scale parameters, respectively. This probability function
can be used to characterize the data set and estimate the extreme pit depth
that possibly can affect the system from which the data was initially produced.
Crevice Corrosion
It occurs at the region of
contact of metals with metals or metals with nonmetals. This is localized
corrosion concentrated in crevices in which the gap is sufficiently wide for
liquid to penetrate into the crevice and sufficiently narrow for the liquid in
the crevice to be stagnant. It may occur at washers, under barnacles, at sand
grains, under applied protective films, and at pockets formed by threaded
joints, beneath flange gaskets, nail and screw heads, in overlap joints,
between tubes and tube plates in heat exchangers etc.
The most typical crevice
corrosion occurs on materials that are passive beforehand, or materials that
can easily be passivated (stainless steels, aluminium, unalloyed or low alloy
steels in more or less alkaline environments etc.), when these materials are
exposed to aggressive species (e.g. chlorides) that can lead to local breakdown
of the surface oxide layer. Materials like conventional stainless steels can be
heavily attacked by deposit corrosion in stagnant or slowly flowing seawater. A
critical velocity of about 2 m/s has often been assumed, but more recent
studies have indicated that crevice corrosion can occur at higher velocities
too.
Crevice corrosion is affected by
several factors, of a metallurgical, environmental, electrochemical, surface
physical, and last but not least, a geometrical nature. One of the most
important factors is the crevice gap. A special form of crevice corrosion that
can develop beneath a protecting film of lacquer, enamel, phosphate or metal is
the so–called filiform corrosion, which leads to a characteristic stripe
pattern. It has been observed most frequently in cans exposed to the
moisturized atmosphere.
Intergranular Corrosion
It is a localized attack on or
adjacent to the grain boundaries of a metal or alloy with insignificant
corrosion on other parts of the surface. This is a dangerous form of corrosion
because the cohesive forces between the grains may be too small to withstand
tensile stresses; the toughness of the material is seriously reduced at a
relatively early stage, and fracture can occur without warning. Grains may fall
out, leaving pits or grooves, but this may not be particularly important.
The general cause of
intergranular corrosion is the presence of galvanic elements due to differences
in concentration of impurities or alloying elements between the material in or
at the grain boundaries and the interior of the grains:
a) Impurities segregated to the
grain boundaries (e.g. the AlFe secondary phase in aluminium).
b) Larger amount of a dissolved
alloying element at the grain boundaries (e.g. Zn in brass).
c) Smaller amount of a dissolved
alloying element at the grain boundaries (e.g. Cr in stainless steel).
In most cases there is a zone of
less noble material in/at the grain boundaries, which acts as an anode, while
the other parts of the surface form the cathode. The area ratio between the
cathode and the anode is very large, and the corrosion intensity can therefore
be high. In some cases, precipitates at the grain boundaries may be more noble
than the bulk material; these precipitates will stimulate grain boundary
attacks by acting as efficient local cathodes (e.g. CuAl2 in aluminium
alloys). Intergranular corrosion occurs in stainless steels and alloys based on
nickel, aluminium, magnesium, copper and cast zinc. In the following sections
we shall look at the three former groups in some detail.
Stress Corrosion Cracking (SCC)
SCC is the crack
formation caused by simultaneous effects of tensile stress and a specific
corrosive environment. SCC is highly chemical specific in that certain alloys
are likely to undergo SCC only when exposed to a small number of chemical environments.
Metal parts with
severe SCC can appear bright and shiny, while being filled with microscopic
cracks. This factor makes it common for SCC to go undetected prior to failure.
SCC often progresses rapidly, and is more common among alloys than pure metals.
The specific environment is of crucial importance, and only very small
concentrations of certain highly active chemicals are needed to produce
catastrophic cracking, often leading to devastating and unexpected failure.
The required
stresses may be due to applied load or in the form of residual stresses from
the manufacturing process, or a combination of both. Cold deformation and
forming, welding, heat treatment, machining and grinding can introduce residual
stresses. The impact of SCC on a material usually falls between dry cracking
and the fatigue threshold of that material.
Usually, most of the surface remains unattacked, but with fine cracks penetrating into the material. In the microstructure, these cracks can have an intergranular or a transgranular morphology. Macroscopically, SCC fractures have a brittle appearance. SCC is classified as a catastrophic form of corrosion, as the detection of such fine cracks can be very difficult and the damage not easily predicted. Experimental SCC data is notorious for a wide range of scatter. A disastrous failure may occur unexpectedly, with minimal overall material loss.
Corrosion Fatigue
Corrosion fatigue is a special
case of stress corrosion caused by the combined effects of cyclic stress and
corrosion. When metals are exposed to the simultaneous actions of corrosive
environment and repeated stress, the fatigue behavior becomes quite different
from that in air and there is a significant decrease in fatigue strength. Thus
this phenomenon is called corrosion fatigue (CF). It is a fatigue in corrosive
environment and should not be confused with SCC.
No metal is immune from some
reduction of its resistance to cyclic stressing if the metal is in a corrosive
environment. Nearly all engineering structures experience some form of alternating
stress, and are exposed to harmful environments during their service life. The
environment plays a significant role in the fatigue of high-strength structural
materials like steel, aluminum alloys and titanium alloys. Materials with high
specific strength are being developed to meet the requirements of advancing
technology. However, their usefulness depends to a large extent on the degree
to which they resist corrosion fatigue.
In corrosion fatigue, the
fatigue-crack-growth rate is enhanced by corrosion. The threshold is lower at
all stress intensity factors. Specimen fracture occurs when the
stress-intensity-factor range is equal to the applicable threshold stress- intensity
factor for stress-corrosion cracking.
Common types of corrosion include
filiform, pitting, exfoliation, intergranular; each will affect crack growth in
a particular material in a distinct way. The degree to which corrosion affects
crack-growth rates also depends on fatigue load levels; for instance, corrosion
can cause a greater increase in crack-growth rates at a low load than it does
at a high load.
Corrosion-fatigue
process is thought to cause rupture of the protective passive film, upon which
corrosion is accelerated. If the metal is simultaneously exposed to a corrosive
environment, the failure can take place at even lower loads and after shorter
time.
In a corrosive
environment the stress level at which it could be assumed a material has
infinite life is lowered or removed completely. Moreover, contrary to a pure
mechanical fatigue, there is no fatigue limit load in corrosion assisted fatigue.
Protection Possibilities Checklist for CF:
i. Minimize or eliminate
cyclic stresses
ii. Reduce stress
concentration or redistribute stress (balance strength and stress throughout
the component)
iii. Select the correct
shape of critical sections
iv. Provide against
rapid changes of loading, temperature or pressure
v. Avoid internal stress
vi. Avoid fluttering and
vibration-producing or vibration-transmitting design
vii. Increase natural
frequency for reduction of resonance corrosion fatigue
viii. Limit corrosion factor in the corrosion-fatigue
process (more resistant material / less corrosive environment).
Fretting Corrosion
It refers to corrosion damage at
the asperities of contact surfaces. Fretting corrosion results from the
combined effects of wear and corrosion and takes place when vibration contact
is made at the interface. In other words, the rapid corrosion that occurs at
the interface between contacting, highly loaded metal surfaces when subjected
to slight vibratory motions is known as fretting corrosion. For fretting
corrosion to occur, the following conditions to be satisfied:
i. Interface must be under load
ii. Relative motion must occur
and should be sufficient enough to produce deformation on the surface
Pits or grooves and oxide debris
characterize this corrosion damage, typically found in machinery, bolted assemblies
and ball or roller bearings. Damage can occur at the interface of two highly
loaded surfaces which are not designed to move against each other. The most
common type of fretting is caused by vibration. The protective film on the
metal surfaces is removed by the rubbing action and exposes fresh, active metal
to the corrosive action of the atmosphere.
Factors affecting fretting
corrosion include contact load, amplitude, frequency, temperature, and
corrosivity of the environment.
Fretting corrosion can be prevented by:
i. Reducing relative movement
between materials
ii. Using materials that are not
susceptible to fretting corrosion
iii. Increasing the hardness of
one or both materials
iv. Using contact lubricants
v. Using seals to absorb
vibrations
Microbial Corrosion
Microbial corrosion (also called
microbiologically - influenced corrosion or MIC) is caused by the presence and activities
of micro-biological organisms or microbes. MIC deteriorates the metal surface
through the metabolic activity of micro-organisms. This process of chiefly acts
on metalloids, metals and rock-based matter.
Biological organisms influence
this type of corrosion. Microbial corrosion is not caused by one microbe, but
can be attributed to several microbes. The common bacteria associated with MIC
are sulfate-reducing bacteria, acid producing bacteria, and iron-reducing
bacteria. Apart from bacteria, microbial corrosion can also be influenced by micro
algae, inorganic and organic chemicals. This influence usually results in a
substantially faster corrosion rate. It affects almost all types of alloys like
stainless, ductile iron and copper, but not titanium. The effect differs among alloys—steel
corrodes faster than ductile iron.
In general, the microbes
responsible for microbial corrosion can be categorized in two groups according
to oxygen requirements:
i. Aerobic (needing oxygen): like
bacteria capable of sulfur oxidizing
ii. Anaerobic (needing no or
little oxygen): like bacteria that are sulfate reducing
Almost all microbial corrosion
takes the appearance of pits forming underneath living matter colonies,
minerals, and bio deposits. This results in a biofilm that results in a
confined environment where the conditions can be corrosive.
This, in turn, hastens the
corrosion process.
The development of microbial corrosion happens in three stages:
i. Microbe attachment (creation
of biofilm)
ii. Growth of initial pit and
nodule (change of environment at the metal surface)
iii. Maturation of nodule and pit
(deterioration of the metal)
Any area collecting stagnant
water or polluted water is very susceptible to microbial corrosion.
Furthermore, micro-organisms that
are capable of utilizing hydrocarbons like pseudomonas aeruginosa can be found
in aviation fuel. This forms dark brown or green mats similar to a gel, and
leads to microbial corrosion on the rubber and plastic parts of the fuel system
of turbine or turbo jet engine.
This corrosion can take many
forms and can be controlled by utilizing mechanical cleaning techniques and
biocides or by conventional corrosion control methods.
Erosion Corrosion (E-C)
It arises from the combined
effect of chemical/electro-chemical attack and physical abrasion as a
consequence of the rapid flow of any turbulent fluid on a metal surface.
Pitting often found on the inner surfaces of pipes is the cause of turbulence. The
rate of erosion increases in turbulent conditions and can result in leakages in
tubes and pipes.
Erosion corrosion can also result
from poor workmanship. When burrs in the tubes are not removed during
installation, these inner burrs cause localized turbulence and hinder the
smooth flow of the fluid. This leads to high rates of pitting in the tubes.
The metal usually has a
protective film, which is the first part to be eroded by the fluid. Once the
film is gone, the bare metal is exposed to corrosion. This type of corrosion is
common in constriction areas. These are areas where there are blockages, inlet
ends, pump impellers as well as other places where there are high rates of
flow.
One form of erosion corrosion is
the cultivation corrosion. This is a special type caused by water bubbles
produced by high-speed impellers. This causes the formation of pits on the
surface of the metal.
Erosion corrosion is more severe
for (i) sour water or seawater on metals at velocities higher than the design
values, (ii) impingement attack by entrained gas bubbles, and/or (iii) abrasion
by water loaded with suspended sand or other solid particulate matter. Such
corrosion is anticipated to be more common during offshore operations. The
metal surface assumes a rough touch and acquires a shiny silver or golden
luster due to the loss of the natural protective film.
Erosion corrosion can be prevented or reduced through any of the following
methods:
i. Reduce the turbulence of the fluid in the tube by
streamlining the piping.
ii. Control the velocity of the fluid to reduce high-flow
velocities.
iii. Use corrosion-resistant materials.
iv. Use corrosion inhibitors and cathodic protection.
v. Ensure that the entire piping system has been de-burred.
vi. Replace sharp angles in the piping system with gentler
angles to avoid constrictions.
vii. Reduce the amount of oxygen dissolved in the fluid.
viii. Adjust the pH value of the fluid.
ix. Change the metal alloy.











My response on my own website. Appreciation is a wonderful thing...thanks for sharing keep it up.
ردحذفNuance PaperPort Professional Crack
DoYourData Uninstaller Pro Crack
Evernote Crack
Lightkey Professional Edition Crack
Ransomware Defender Crack
Qimage Ultimate Crack
GOM Player Plus Crack
Urban VPN Crack
v
ردحذف