Carbon credits are a tradable permit scheme. It is a simple, non-compulsory way to counteract the greenhouse gasses that contribute to climate change and global warming. Carbon credits create a market for reducing greenhouse emissions by giving a monetary value to the cost of polluting the air. The Carbon Credit is this new currency and each carbon credit represents one tonne of carbon dioxide either removed from the atmosphere or saved from being emitted. Carbon credits are also called emission permit. Carbon credit is in the Environment and Pollution Control subject. Carbon credits are certificates awarded to countries that are successful in reducing emissions of greenhouse gases.
Generation of Carbon Credits
Carbon credits are generated as the result of an additional carbon project. Carbon credits can be created in many ways but there are two broad types:
1. Sequestration (capturing or retaining carbon dioxide from the atmosphere) such as afforestation and reforestation activities.
2. Carbon Dioxide Saving Projects such as use of renewable energies
These credits need to be authentic, scientifically based and Verification is essential. Carbon credit trading is an innovative method of controlling emissions using the free market.
Need for Carbon Credits
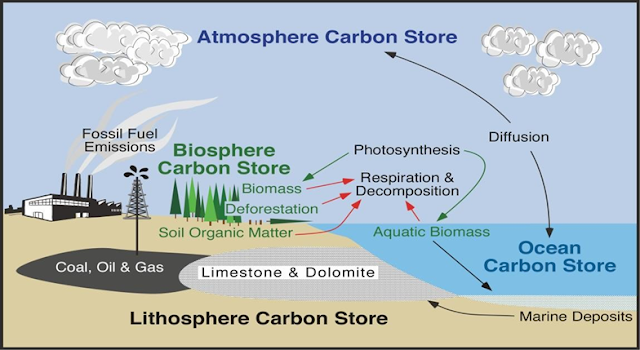
Over millions of years, our planet has managed to regulate concentrations of greenhouse gases through sources (emitters) and sinks (reservoirs). Carbon (in the form of CO2 and methane) is emitted by volcanoes, by rotting vegetation, by burning of fossil fuels and other organic matter. But CO2 is absorbed, by trees, forests or by some natural phenomenon like photosynthesis and also oceas to some extent.
Types of Carbon Credit
There are two main markets for carbon credits:
A) Compliance Market credits
B) Verified Market credits (VERs)
Value of Carbon Credits
Carbon credits create a market for reducing greenhouse gases emissions by giving a monetary value to the cost of polluting the air such as carbon emitted by burning of fossil fuels. This means that carbon becomes a cost of business and is seen like other inputs such as raw materials or labor.
Carbon credits are measured in tonnes of carbon dioxide.
1 credit = 1 tonne of CO2.
Each carbon credit represents one metric ton of C02 either removed from the atmosphere or saved from being emitted. The carbon credit market creates a monetary value for carbon credits and allows the credits to be traded.
For each tonne of carbon dioxide that is saved or sequestered carbon credit producers may sell one carbon credit.
Carbon Sequestration or Carbon Capture and Storage or Scrubbing of CO2
Carbon sequestration is the capture of carbon dioxide (CO2) and may refer specifically to:
• "The process of removing carbon from the atmosphere and depositing it in a reservoir." When carried out deliberately, this may also be referred to as carbon dioxide removal, which is a form of geoengineering.
• The process of carbon capture and storage, where carbon dioxide is removed from flue gases, such as on power stations, before being stored in underground reservoirs.
• Natural biogeochemical cycling of carbon between the atmosphere and reservoirs, such as by chemical weathering of rocks.
Carbon sequestration describes long-term storage of carbon dioxide or other forms of carbon to either mitigate or defer global warming and avoid dangerous climate change. It has been proposed as a way to slow the atmospheric and marine accumulation of greenhouse gases, which are released by burning fossil fuels.
Carbon dioxide is naturally captured from the atmosphere through biological, chemical or physical processes. Some anthropogenic sequestration techniques exploit these natural processes, while some use entirely artificial processes.
Carbon dioxide may be captured as a pure by-product in processes related to petroleum refining or from flue gases from power generation.CO2 sequestration includes the storage part of carbon capture and storage, which refers to large-scale, permanent artificial capture and sequestration of industrially produced CO2 using subsurface saline aquifers, reservoirs, ocean water, aging oil fields, or other carbon sinks.
Steps
A) Capturing or Scrubbing
B) Transporation
C) Sequestration or Storage
A) CAPTURING or SCRUBBING OF CO2
TECHNOLOGIES:
Broadly, three different types of technologies for scrubbing of CO2 exist:
1. post-combustion,
2. pre-combustion, and
3. oxyfuel combustion
4. Chemical looping
5. Calcium Looping
1. Post-Combustion: In post combustion capture, the CO2 is removed after combustion of the fossil fuel — this is the scheme that would be applied to fossil-fuel burning power plants. Here, carbon dioxide is captured from flue gases at power stations or other large point sources. The technology is well understood and is currently used in other industrial applications, although not at the same scale as might be required in a commercial scale power station.
2. Pre-Combustion : The technology for pre-combustion is widely applied in fertilizer, chemical, gaseous fuel (H2, CH4), and power production. In these cases, the fossil fuel is partially oxidized, for instance in a gasifier. The resulting syngas (CO and H2O) is shifted into CO2 and more H2. The resulting CO2 can be captured from a relatively pure exhaust stream. The H2 can now be used as fuel; the carbon dioxide is removed before combustion takes place. There are several advantages and disadvantages when compared to conventional post combustion carbon dioxide capture. The CO2 is removed after combustion of fossil fuels, but before the flue gas is expanded to atmospheric pressure. This scheme is applied to new fossil fuel burning power plants, or to existing plants where re-powering is an option. The capture before expansion, i.e. from pressurized gas, is standard in almost all industrial CO2 capture processes, at the same scale as will be required for utility power plants.
3. Oxy-Fuel Combustion: In oxy-fuel combustion the fuel is burned in oxygen instead of air. To limit the resulting flame temperatures to levels common during conventional combustion, cooled flue gas is re-circulated and injected into the combustion chamber. The flue gas consists of mainly carbon dioxide and water vapor, the latter of which is condensed through cooling. The result is an almost pure carbon dioxide stream that can be transported to the sequestration site and stored. Power plant processes based on oxy fuel combustion are sometimes referred to as "zero emission" cycles, because the CO2 stored is not a fraction removed from the flue gas stream (as in the cases of pre- and post-combustion capture) but the flue gas stream itself. A certain fraction of the CO2 generated during combustion will inevitably end up in the condensed water. To warrant the label "zero emission" the water would thus have to be treated or disposed of appropriately. The technique is promising, but the initial air separation step demands a lot of energy.
4. Chemical looping combustion (ClC): Chemical looping uses a metal oxide as a solid oxygen carrier. Metal oxide particles react with a solid, liquid or gaseous fuel in a fluidized bed combustor, producing solid metal particles and a mixture of carbon dioxide and water vapor. The water vapor is condensed, leaving pure carbon dioxide which can then be sequestered. The solid metal particles are circulated to another fluidized bed where they react with air, producing heat and regenerating metal oxide particles that are re circulated to the fluidized bed combustor.
5. Calcium looping: A variant of chemical looping is calcium looping, which uses the alternating carbonation and then calcinations of a calcium oxide based carrier as a means of capturing CO2.
B) TRANSPORT
After capture, the CO2 would have to be transported to suitable storage sites. This is done by pipeline, which is generally the cheapest form of transport. In 2008, there were approximately 5,800 km of CO2 pipelines in the United States, used to transport CO2 to oil production fields where it is then injected into older fields to extract oil. The injection of CO2 to produce oil is generally called Enhanced Oil Recovery or EOR.
In addition, there are several pilot programs in various stages to test the long-term storage of CO2 in non-oil producing geologic formations.
A COA conveyor belt system or ship could also be utilized for transport. These methods are currently used for transporting CO2 for other applications.
C) SEQUESTRATION or STORAGE
Various forms have been conceived for permanent storage of CO2. These forms include gaseous storage in various deep geological formations (including saline formations and exhausted gas fields), liquid storage in the ocean, and solid storage by reaction of CO2 with metal oxides to produce stable carbonates.
i) GEOLOGICAL STORAGE
Also known as geo-sequestration, this method involves injecting carbon dioxide, generally in supercritical form, directly into underground geological formations. Oil fields, gas fields, saline formations, unmineable coal seams, and saline-filled basalt formations have been suggested as storage sites. Various physical (e.g., highly impermeable cap rock) and geochemical trapping mechanisms would prevent the CO2 from escaping to the surface.
Enhanced oil recovery:CO2 is sometimes injected into declining oil fields to increase oil recovery. This option is attractive because the geology of hydrocarbon reservoirs is generally well understood and storage costs may be partly offset by the sale of additional oil that is recovered. Disadvantages of old oil fields are their geographic distribution and their limited capacity, as well as the fact that subsequent burning of the additional oil so recovered will offset much or all of the reduction in CO2 emissions.
Unmineable coal seams can be used to store CO2 because the CO2 molecules attach to the surface of coal. The technical feasibility, however, depends on the permeability of the coal bed. In the process of absorption the coal releases previously absorbed methane, and the methane can be recovered (enhanced coal bed methane recovery). The sale of the methane can be used to offset a portion of the cost of the CO2 storage. Burning the resultant methane, however, would produce CO2, which would negate some of the benefit of sequestering the original CO2.
II) OCEAN STORAGE
Another proposed form of carbon storage is in the oceans. Several concepts have been proposed:
• 'Dissolution' injects CO2 by ship or pipeline into the ocean water column at depths of 1000 – 3000 m, forming an upward-plume, and the CO2 subsequently dissolves in seawater.
• Through 'lake' deposits, by injecting CO2 directly into the sea at depths greater than 3000 m, where high-pressure liquefies CO2, making it denser than water, and forms a downward-plume that may accumulate on the sea floor as a 'lake', and is expected to delay dissolution of CO2 into the ocean and atmosphere, possibly for millennia.
• Use a chemical reaction to combine CO2 with a carbonate mineral (such as limestone) to form bicarbonate(s), for example: CO2 + CaCO3 + H2O → Ca(HCO3)2(aq). However, the aqueous bicarbonate solution must not be allowed to dry out, or else the reaction will reverse.
• Store the CO2 in solid clathrate hydrates already existing on the ocean floor,[23][24] or growing more solid clathrate.
The environmental effects of oceanic storage are generally negative, and poorly understood. Large concentrations of CO2 could kill ocean organisms, but another problem is that dissolved CO2 would eventually equilibrate with the atmosphere, so the storage would not be permanent.In addition, as part of the CO2 reacts with the water to form carbonic acid, H2CO3, the acidity of the ocean water increases.
The bicarbonate approach would reduce the pH effects and enhance the retention of CO2 in the ocean, but this would also increase the costs and other environmental effects.
III) MINERAL STORAGE
In this process, CO2 is exothermically reacted with available metal oxides, which in turn produces stable carbonates. This process occurs naturally over many years and is responsible for a great amount of surface limestone. The reaction rate can be made faster, for example by reacting at higher temperatures and/or pressures, or by pre-treatment of the minerals, although this method can require additional energy.
Carbon sequestration by reacting naturally occurring Mg and Ca containing minerals with CO2 to form carbonates has many unique advantages. Most notable is the fact that carbonates have a lower energy state than CO2, which is why mineral carbonation is thermodynamically favorable and occurs naturally (e.g., the weathering of rock over geologic time periods). Secondly, the raw materials such as magnesium based minerals are abundant. Finally, the produced carbonates are unarguably stable and thus re-release of CO2 into the atmosphere is not an issue. However, conventional carbonation pathways are slow under ambient temperatures and pressures. The significant challenge being addressed by this effort is to identify an industrially and environmentally viable carbonation route that will allow mineral sequestration to be implemented with acceptable economics.